Fujitsu Tackles ‘Drug Loss’ in Japan through Ecosystem to Accelerate Digitalization of Clinical Trials




FUJITSU forms strategic partnership with Paradigm and will provide offerings that leverage the power of AIFujitsu Limited
Kawasaki, August 26, 2024
Fujitsu today announced that it will begin initiatives to attract global clinical trials to Japan and tackle the ‘drug loss’ issue by working with pharmaceutical companies and medical institutions to build a new medical data ecosystem. The efforts come under the Fujitsu Uvance Healthy Living, which aims to improve people’s well-being.
In July 2024, Fujitsu formed a strategic partnership with Paradigm Health, Inc., a US startup that provides one of the world’s most advanced clinical trial platforms. Through utilizing this platform, Fujitsu’s Healthy Living Platform, and the Fujitsu Kozuchi AI service, Fujitsu and Paradigm will facilitate the collection and processing of data stored at medical institutions and speed up the clinical trial planning process.
In addition, starting today, Fujitsu will release Patient-centric Clinical Trials, a new service for automatically creating clinical trial documents using Fujitsu’s proprietary large language model (LLM). This service will be positioned in the company’s Healthy Living portfolio.
Fujitsu will continue to expand its support to include not only clinical trial planning, but also implementation, and will work to solve issues throughout the entire clinical trial process. Through these efforts, Fujitsu aims to secure 20.0 billion yen in revenue in fiscal 2030, as well as contribute to achieving a society in which patients can quickly obtain the medicine they need and choose the treatment that best suits them.
Background
In Japan, patients who participate in clinical trials are dispersed across multiple hospitals making the process costly and time consuming. There has been an increasing number of cases in which Japan is excluded from regions targeted for global clinical trials. The impact of Japan’s drug pricing policy is also contributing to this situation.
As a result, the number of drugs that are in use outside of Japan but are not approved for use in Japan had risen to 143 as of March 2023 (source: the Japanese Ministry of Health, Labour and Welfare of Japan).
Overview of the Initiatives
1. Accelerating Digitization in the Area of Clinical Trials through Fujitsu’s Partnership with Paradigm
Fujitsu will work with Paradigm, a US startup that provides pharmaceutical companies and medical institutions with one-stop support for the clinical trial process, to develop a new clinical trial data ecosystem that utilizes medical data. Paradigm’s clinical trial platform, which hosts many global clinical trials and has a long track record of success, will be combined with Fujitsu’s operational support expertise and cutting-edge technologies in the area of healthcare and life sciences in Japan.
Clinical data, such as medical data and genomic data, will be collected from medical institutions through Fujitsu’s Healthy Living Platform. Fujitsu’s AI service, Fujitsu Kozuchi, will process this data so that it is compliant with the necessary laws and regulations, and it will be provided to Paradigm. In addition, Paradigm will use its clinical trial platform to analyze the data and provide pharmaceutical companies with the insight needed to plan and conduct clinical trials. This will allow pharmaceutical companies to better understand the situation at the medical institutions as well as the distribution of patients during the planning phase of the clinical trial, and significantly optimize the clinical trial design process.
Medical institutions will also be able to quickly access information about clinical trials in which patients are able to participate through Paradigm’s clinical trial platform, making it easier for medical institutions to encourage patients to participate in clinical trials at the appropriate time.
Fujitsu will launch these solutions starting in September 2024, beginning with providing support to core clinical research hospitals.
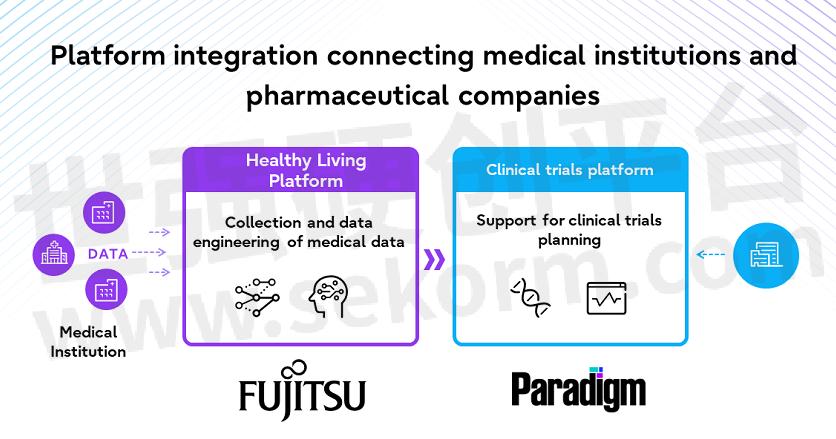
Overview of the platform integration connecting medical institutions and pharmaceutical companies
2. Providing Patient-centric Clinical Trials, a New Offering for Optimizing Clinical Trial Workflows through a Specialized LLM
In Japan, the hundreds of clinical trial-related documents that pharmaceutical companies need to produce to develop a new drug are still being created and managed manually. However, by using its LLM specialized for clinical trials, Fujitsu will offer a service that can automatically create these documents and ensure that they are in compliance with regulations. This service will be provided in Japan as the Patient-centric Clinical Trials offering as part of its Healthy Living portfolio.
In conducting a PoC of this offering with a pharmaceutical company, the LLM was able to automatically create approximately 80% of each document. According to Fujitsu’s estimates, this is expected to lead to a reduction in the total time required to create documentation of up to 50%. Through this offering, Fujitsu aims to make a significant contribution to optimizing the clinical trial planning and implementation workflow.
Future Plans
Fujitsu will work to accelerate the digitalization of the clinical trial environment in Japan by providing total support for the entire clinical trial process, including planning and implementation. Through these efforts, Fujitsu aims to create the groundwork for increasing the number of global clinical trials conducted in Japan, solve the issue of drug loss and contribute to achieving a society in which all individuals can choose to live their lives in the way that suits them.
Statement from Kent Thoelke, CEO of Paradigm Health, Inc.
Paradigm is excited to be partnering with Fujitsu to maximize clinical trial efficiency in Japan. This collaboration will modernize the clinical trial model and provide unprecedented access to clinical trials for patients across Japan. The partnership will leverage Paradigm’s industry leading Platform and Fujitsu’s expansive healthcare solutions to increase clinical trial patient accrual, decrease overall drug development timelines and costs, while working to eliminate drug loss in Japan. We believe that this collaboration will establish Japan as an essential part of the global clinical trials and drug development ecosystem while ensuring that Japanese patients have equitable access to the best possible care options.
- |
- +1 赞 0
- 收藏
- 评论 0
本文由JWM转载自Fujitsu Official Website,原文标题为:Fujitsu tackles ‘drug loss’ in Japan through ecosystem to accelerate digitalization of clinical trials,本站所有转载文章系出于传递更多信息之目的,且明确注明来源,不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
相关推荐
Fujitsu Achieves Major Technical Milestone with World‘s Fastest 36 Aubit Quantum Simulator
Fujitsu has successfully developed the world‘s fastest quantum computer simulator capable of handling 36 qubit quantum circuits on a cluster system featuring Fujitsu’s “FUJITSU Supercomputer PRIMEHPC FX 700“ (“PRIMEHPC FX 700”).
Fujitsu Components Selects Wirepas Mesh for Advanced IoT Solution Development
As a leading company in wireless modules, beacons, and sensor devices, Fujitsu Components has added several complementary technologies to simplify data collection over various wireless technologies from sensor fitted beacons, making IoT connectivity a simple to integrate exercise.
Fujitsu Limited and Fujitsu Australia today announced acquisition plans for Asia-Pacific region’s largest independent ServiceNow-specialist, Enable Professional Services
Fujitsu Limited and Fujitsu Australia today announced acquisition plans for the Asia-Pacific region’s largest independent ServiceNow specialist, Enable Professional Services.
案例研究富士通-Numazu云中心
描述- Fujitsu Limited通过在Numazu建立软件开发云中心,实现了对服务器资源的集中管理和虚拟化,提高了开发环境的部署效率。通过标准化操作流程和自动化部署,Fujitsu成功缩短了开发环境搭建时间,降低了运营成本,并提高了资源利用率。该项目使Fujitsu的软件开发活动更加高效,并带来了显著的财务和环境效益。
FUJITSU(富士通)铁电随机存储器 FRAM 产品概览(中文)
目录- FRAM特长与产品阵容 FRAM热点产品 独立FRAM存储器 FRAM内置RFID用LSI
型号- MB85RDP16L,MB85RS128B,MB97RXXXX,MB85R4002A,MB85RS16N,MB97R8050,MB85RS16,MB89R112AHF,MB85R256F,MB89R118CHF,MB89R119B,MB97R804B,MB85RS256B,MB85R1002A,MB85RC16,MB85RC512T,MB85RC1MT,MB85RC64V,MB85RS64,MB85R1001,MB85RDP16LX,MB85R4001A,MB85RS512T,MB85RC64TA,MB85R4001,MB85RC128A,MB94R330,MB85RC64A,MB89R112A,MB85R4M2T,MB89R118C,MB85RC256V,MB85RQ4ML,MB97R803A,MB85RC04V,MB85RS2MT,MB85RC16V,MB89R119BHF,MB85RS1MT,MB85RS64V,MB85R1001A
穆尔西亚天主教大学富士通提升UCAM IT基础设施
描述- UCAM大学与Fujitsu合作,通过AWS云迁移和灾难恢复(DR)服务,提升了其关键基础设施服务的可靠性和安全性。Fujitsu部署了基于云的DR解决方案,包括多因素认证、安全组和NACLs,确保了大学服务的持续可用性和数据中心的备份。该项目缩短了灾难恢复时间,提高了安全性,并促进了大学向云环境的转型。
【选型】FUJITSU(富士通)继电器(信号继电器、功率继电器、EV/PHV继电器)选型指南
目录- 汽车继电器 EV/PHV继电器 功率继电器 信号继电器
型号- JV SERIES,FTR-K2W,FTR-K3-PS,JY SERIES,FTR-B3-AUT,JS SERIES,FTR-E1,FTR-LY,FTR-E3,NY SERIES,FBR53-HW,FTR-F1L,FTR-K3L,FBR582,SY SERIES,FTR-P7,FTR-H2,FTR-P2,FTR-K2G,NA,FTR-P3,FTR-F4G,FTR-P4,FTR-P5,FTR-P6,FTR-K3-PV,FBR51NL,FTR-K1-HC,FTR-C2,FBR57,FBR572,FBR59-EV,RY,FTR-K2,FTR-K3,JS,FTR-G1,FTR-K5,NY,JV,FBR53,JY,FTR-C1,FTR-S2,FTR-K1,FTR-K1L,RY SERIES,FBR59-HW,FTR-K3-WS,FTR-B3,FTR-B4,SY,NA SERIES,FTR-F3-OM3,FTR-J2,FTR-F1,FTR-V1M,FTR-F2,FTR-MY,FTR-F3,FTR-F4,JS-RW,FTR-K1-500,FTR-K3-WG,FTR-V1
富士通智能集成商与甲骨文解决方案
描述- Fujitsu Smart Integrator解决方案旨在帮助客户实现“云之旅”,通过提供“Any-to-Any”集成能力,连接Oracle SaaS Cloud与Oracle R12EBS(本地或OCI)。该方案包括预构建的集成,使企业能够灵活采用“最佳组合”的混合方法,实现业务功能的现代化。Fujitsu提供从业务流程分析、设计映射到部署的全方位服务,并确保云迁移过程平滑、连续。
Fujitsu(富士通)与世强的代理授权书
描述- 本文件由富士通组件香港有限公司发出,授权世强先进(深圳)科技股份有限公司或世强有限在中国市场推广和销售富士通组件产品。文件保证通过上述公司提供的富士通组件产品符合富士通组件有限公司的质量标准。授权信有效期为一年。
富士通壁画PoS解决方案的主要特点
描述- Fujitsu Fresco PoS解决方案提供全面的一站式零售点解决方案,包括前端结账、服务台/退货、店内熟食/咖啡厅服务、自助结账、自助服务亭、现金自动化和工作任务管理技术。该解决方案具备实时系统监控和管理、自动化软件更新和部署、数字人类学UI/UX设计、物联网集成、免提现金管理自动化、药房系统集成、客户忠诚度管理、电子福利转移(EBT)和e-WIC交易支持、复杂促销管理、安全支付处理以及与企业实时销售数据连接等功能。
长沙市卫生局案例研究
描述- 长沙县卫生局通过信息化建设,构建了基于Fujitsu PRIMERGY服务器的HIS系统,实现了医疗和健康记录的整合,提高了医疗服务质量和效率,降低了管理成本,并促进了管理方法创新。该系统不仅提升了患者满意度和忠诚度,还支持了医院业务发展,实现了资源优化配置,提高了医院管理能力和盈利水平。
型号- PRIMERGY RX600,RX300 S6,PRIMERGY RX300 S6,RX600 S6,PRIMERGY RX300,PRIMERGY RX600 S6,ETERNUS DX80,DX80,DX80 S2
Fujitsu TP8 Modular Point of Service Terminal
描述- Fujitsu TP8 Modular PoS是一款面向零售商的高性能、多功能点服务终端。它采用英特尔第六代和第七代处理器,配备快速M.2 PCIe NVMe SSD存储,支持从入门级到高端的各种商店格式。该设备具有超紧凑的设计,可适应各种环境,并提供高达18个USB端口和3个串行端口。TP8 Modular结合了领先的性能、外围和网络连接,同时提供灵活的部署选项和降低的支持成本。
型号- I3-7101E,FP-2000,G3930E,FP-2200,FP-2100,I5-7500,G3900,TM-T88V/V1,I5-6500,FP510II,I3-6100,TP8,AC97
富士通组件无线模块
描述- Fujitsu Component Limited 发布了2019年2月的无线产品更新,涵盖了蓝牙、低功耗广域网(LPWA)、超高频传感器和2.4GHz网状网络产品线。主要内容包括关键技术和能力、产品路线图、Fujitsu无线模块的优势,以及各种模块的详细规格和应用。产品线包括不同版本的蓝牙模块、低功耗广域网模块、超高频传感器模块和2.4GHz网状网络模块,适用于各种应用场景。
型号- FWM8BLZ02B-109079,MBH7BTZ43,MBH7BTZ42,MBH7BTZ40,MBH7BLZ01,MBH7BLZ02,BLZ01A -*,MBH7BLZ02A,BTZ40,BTZ43,MBH7BTZ42A,BTZ42,FWM-ORCA-EVB1,FWM8BLZ02-109042,BLZ07,BLZ01,MBH7BLZ01A-1090**,MBH7BLZ07,BLZ02,BLZ02A-1090**,FWM7SLZ02A,FWM7BLZ20-EVB2-EF2,BLZ07-*,MBH7BLZ01A,FWM8BLZ02A-109047,FWM7BLZ20B-109077,MBH7BLZ07-1090**,BLZ02-1090**,BLZ01-*,BTZ39A,BLZ02A-*,MBH-SARDINE-EVB,MBH-SAKURA2-E,MBH7BLZ01-1090**,FWM7BLZ20-1090**,MBH7BLZ02A-1090**,FWM7BLZ20B,FWM7BLZ20,BTZ42A,BLZ02A,BLZ01A-1090**,BLZ07-1090**,FWM7BLZ20-EVB2-EB2,MB7BLZ07,FWM7BLZ20W,FWM8BLZ03Y,FWM8BLZ03W,MBH7BTZ39A,BTZ39,FWM7RAZ01,FWM8BLZ02A-109069,MBH7BTZ39,BLZ01-1090**,MBH7BLZ02-1090**,MBH-FUJI2-E1,BLZ02-*
电子商城

































































































































































































登录 | 立即注册
提交评论