Root Cause Analysis on Return Merchandise Authorization (RMA) Boards




Every manufacturing company strives to produce 100% passing boards with good quality. With Industry 4.0 transformation, there are now more readily available data analytics to accelerate the effort towards this ultimate goal.Now KEYSIGHT will talk about Root Cause Analysis on Return Merchandise Authorization (RMA) Boards.
It's common understanding that only passing boards in the production line get shipped out from factory to the market. However, in some rare occasions, products or boards failed earlier than expected in the market. And these early failure boards are returned to the factory for diagnostics; these boards are also often known as Return Merchandise Authorization (RMA) boards.
Within PathWave Manufacturing Analytics application, there is an RMA module which allows user to retrieve back test history of a board based on its serial number. Identifying the issue for this early failure is important, but even more importantly is to identify potential quality risk of other similar boards out there in the market and to prevent such quality gaps in future. As this may have diverse impact on company reputation.
Once the serial number of the early failure board is entered into the RMA module, test history on this board will be retrieved and displayed as shown in Figure 1.
The test history will provide information like how many times this board had been tested with details including the Start and End Time, which fixture and equipment the board had tested on, together with its Pass/Fail status. This allows traceability of the early failure boards.
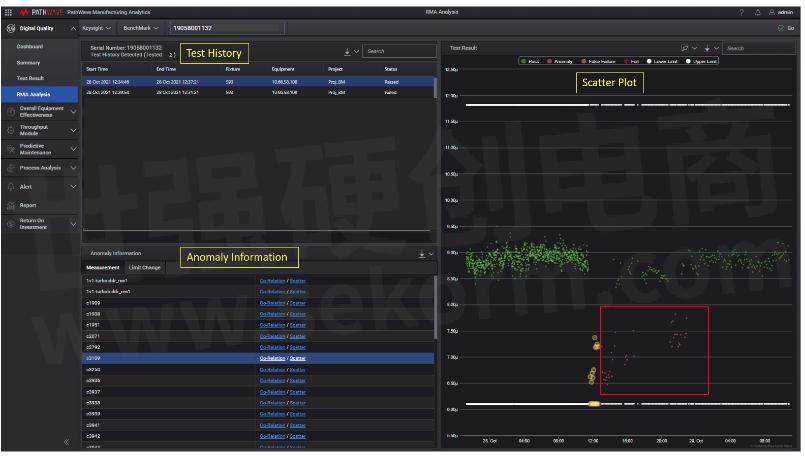
Figure 1: RMA - Measurement
Depending on which run is selected, the corresponding measurement anomaly, limit change and failure information (if any) will be displayed in the Anomaly Information section (see Figure 1).
In this example, there are multiple measurement anomalies detected during the run. And for each measurement anomaly, its corresponding measurement trend is displayed in the scatter plot on the right. The serial number board is highlighted in yellow. Noted that a measurement anomaly is not a failing measurement. In fact, it is a passing measurement within the limits; however, it is detected as an outlier from the population based on PMA anomaly detection model. A measurement anomaly (which is a passing measurement) is definitely going to be overlook as compared to a failed measurement.
Highlighting measurement anomaly here will definitely point user to a reasonable starting point of root cause analysis. Being an outlier from the population may have higher chances of causing board functionality to go out of specifications. From the opposite angle, not finding any measurement anomaly on highly possible test (like components related directly to the early failure), will indicate to user that other external factors may need to be considered.
At the same time, further tracking and investigation can be performed on other boards which exhibit similar measurement anomaly as highlighted in red box in Figure 1; to determine if they pose potential risk out there in the market.
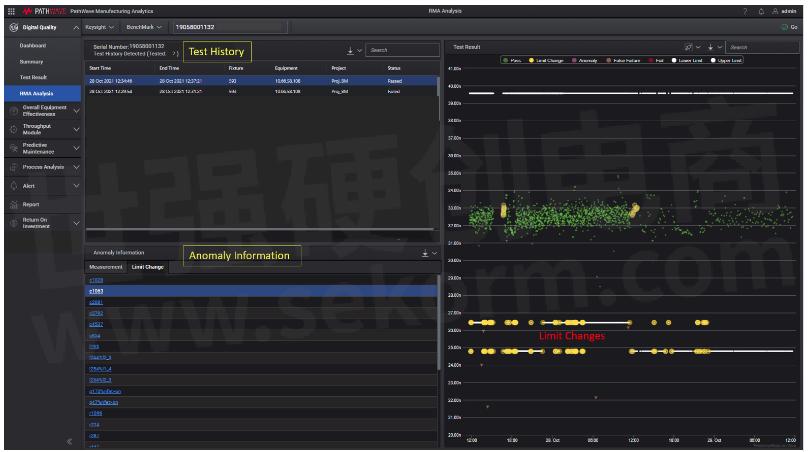
Figure 2: RMA - Limit Change
In Figure 2, under the Limit Change tab, PMA will highlight any limit change detected during the period when board was tested as unauthorized limit change may pose potential risk to product quality. One can imagine the catastrophic impact when test limits have been altered incorrectly and a supposing failed board escaped out into the market, especially for live threating products in medical and automotive markets.

Figure 3: RMA - Failure
In Figure 3, under the Failure tab, PMA will display test failures associated to the board for the run. This helps user understand what failures and repairs have occurred previously for the board.
These information allow user to perform root cause analysis on this early failure board holistically.
- |
- +1 赞 0
- 收藏
- 评论 0
本文由董慧转载自Keysight,原文标题为:Root Cause Analysis on Return Merchandise Authorization (RMA) Boards,本站所有转载文章系出于传递更多信息之目的,且明确注明来源,不希望被转载的媒体或个人可与我们联系,我们将立即进行删除处理。
相关研发服务和供应服务
相关推荐
Automotive Relay Storage Environment
MEISHUO will introduce the automotive relay storage environment in this article.
Choosing The Right DC-DC Converter For Your Critical Medical Application
P-DUKE’s MPQ60W medical-grade DC-DC converter is rated for the most stringent CF-type medical devices with less than 4.5μA of leakage current, 5000VAC of isolation, reinforced 2xMOPP insulation, and 8 mm of air clearance and creepage distance.
PathWave Manufacturing Analytics (PMA) Use Case: Faster diagnostics for Semiconductor recalls
Keysight has seen car recalls due to faulty semiconductor components. Within the PMA application, there is an RMA module that allows a user to retrieve the complete test history of the board and its batch.
New 2W medical grade DC/DC converter in a compact SIP-8 package
For the ever-increasing demand for medical home care and medical equipment, P-DUKE has extended its medical power conversion portfolio with a MPL02 board-mount DC/DC converter series. The MPL02 series is designed especially for compact medical applications and comes in a tiny SIP-8 industrial standard package (footprint only 9.9 x 21.8 mm) which saves space on the PCB.
ELETE Press-fit With High Electrical Reliability, Suitable For Communications, Automotive Electronics, Medical And Other Industries
Press-fit technology is an alternative solution to weld mounting. The reliable connection of electrical contacts is realized mainly through the non-welding method of cold connection.The Press Fit end is pressed into the PCB hole to obtain a higher clamping force with a smaller pressing force. During pressing, it deforms elastically and provides tight connections with low contact impedance and high reliability.Because of its high electrical reliability, ELETE Press-fit are widely used in communications, automotive electronics, medical and other industries.
PORON Medical® Urethanes – Slow Recovery Typical Physical Properties
描述- 本资料介绍了Rogers Corporation旗下的Poron Medical品牌的聚氨酯泡沫材料,提供了其典型的物理性质数据。内容包括密度、压缩回弹率、硬度、耐水解性、抗微生物性、皮肤接触安全性和化学耐受性等。
型号- PORON MSRVF,PORON MSRVS,PORON MSRF,PORON MEDICAL®,PORON MSRS,PORON MSRF .PORON MSRVF
PORON Medical® Urethanes – Firm – Energy AbsorbingTypical Physical Properties
描述- 本资料介绍了Rogers Corporation旗下的Poron Medical品牌的聚氨酯泡沫材料。该材料具有高能量吸收能力,适用于医疗设备等领域。
型号- PORON MF,PORON MEDICAL®
Semiconductor Peltier In Medical Application
In fact, the application of semiconductor Peltier is very strict. In view of this point, today we will explain to you the medical application of thermoelectric Peltier.
Environment Factors and the Right Tool to Weight in Medical Sensor Design
In fact, when it comes to creating a sensor that fulfills its purpose in patient care without worry, environmental considerations are one of the most important design elements to discuss with your sensor manufacturer.
Medical Power Series: An Innovative Line of Medical Equipment that has Revolutionized the Healthcare Industry
The Medical Power Series is an innovative line of medical equipment that has revolutionized the healthcare industry. The Medical Power Series includes a range of products, each with their unique features and benefits.
What Are the Points to Consider When Choosing an Automotive Connector?
To figure out the next generation of vehicle connectivity solutions, the following must be considered: the size and power supply, the performance and specifications, the wire size range, and the flexibility of automotive connectors.
Amphenol Custom OEM Sensors for Medical Devices & Your Bottom Line
Amphenol custom OEM sensors for medical devices offer the perfect blend of sophistication and strength that you need to optimize your device‘s performance with reliable and robust technology.
P-Duke MPU02 Series 2W Medical-grade DC/DC Converters in a SIP-9 Casing, with Only 8mm Clearance/ Creepage Distances
With the MPU02 series, P-Duke has extended its high-performance range of medical-grade DC/DC converters from 1W to 60W. MPU02 series DC/DC modules have 2MOPP Means of Patient Protection, low 2μA leakage current, and 5000VAC I/O-isolation voltage.
Poron Medical®聚氨酯产品安全信息表
描述- 本资料为Poron Medical® Urethanes(Poron医疗级聚氨酯)的产品安全信息表。资料内容包括产品标识、危害识别、成分信息、急救措施、消防措施、意外泄漏处理、个人防护、物理和化学性质、稳定性与反应性、毒理学信息、生态信息、处置考虑、运输信息、法规信息和其他信息。资料强调正常使用条件下,该产品不会释放有害化学物质,因此无需安全数据表。
型号- PORON MEDICAL®
PORON Medical Urethanes – Soft Cushioning & Soft Supporting Materials
描述- 该资料介绍了Rogers Corporation旗下的Poron Medical品牌的软质聚氨酯泡沫材料。这些材料具有中等密度、微细孔结构,适用于骨科和假肢应用,包括定制矫形器、预制矫形器、假肢衬垫和其他生物力学支撑。
型号- PORON MEDICAL
现货市场
服务
提供是德(Keysight),罗德(R&S)测试测量仪器租赁服务,包括网络分析仪、无线通讯综测仪、信号发生器、频谱分析仪、信号分析仪、电源等仪器租赁服务;租赁费用按月计算,租赁价格按仪器配置而定。
提交需求>
朗能泛亚提供是德(Keysight),罗德(R&S)等品牌的测试测量仪器维修服务,包括网络分析仪、无线通讯综测仪、信号发生器、频谱分析仪、信号分析仪、电源等仪器维修,支持一台仪器即可维修。
提交需求>


































































































































































































登录 | 立即注册
提交评论